Management Techniques
Management Techniques
Up to 94% of patients experience side effects while taking endocrine therapy, with up to 18% of them discontinuing therapy due to treatment-related adverse events.1
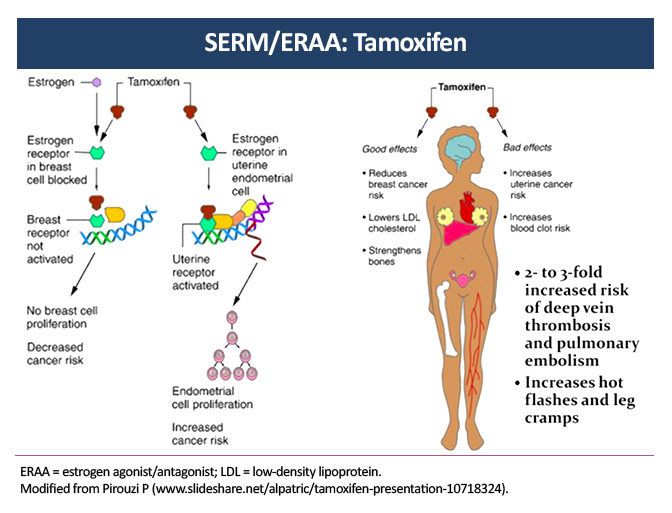
Selective Estrogen Receptor Modulator: Adverse-Event Overview
Hot flashes affect up to 80% of patients taking the selective estrogen receptor modulator (SERM) tamoxifen and are one of the most common and bothersome side effects of endocrine therapy. Moderate cytochrome P450 2D6 (CYP2D6) inhibitors, such as sertraline and duloxetine, have been shown to minimize the side effects of tamoxifen without affecting its efficacy. Strong CYP2D6 inhibitors, such as paroxetine and fluoxetine, are not recommended.3
The relative risk of venous thromboembolism (VTE) is increased by a factor of 2–3 in older women receiving tamoxifen. Risk factors for VTE include extended therapy (10 years of tamoxifen instead of 5 years), prior surgery, fracture, and immobilization.4
Tamoxifen has been associated with an increased risk of endometrial cancer and uterine sarcoma that is increased by a factor of 2.7 during use and declines after discontinuation. It is recommended that premenopausal women with any irregular bleeding or postmenopausal women with new-onset vaginal bleeding should be evaluated by hysteroscopy or endometrial biopsy.5
Other common side effects of the SERM tamoxifen include cold sweats, night sweats, vaginal discharge, bleeding, dryness, genital itching, and sexual dysfunction.6 A routine annual eye examination is recommended for all patients receiving tamoxifen due to the risk of cataracts, macular edema, and vision loss.7
Aromatase Inhibitors
Estrogen deficiency is a risk factor for osteoporosis. The use of aromatase inhibitors (AIs) is associated with a significantly increased risk of bone fractures compared with tamoxifen in postmenopausal women with early-stage breast cancer (odds ratio of 1.47).8 National Comprehensive Cancer Network (NCCN) guidelines recommend an evaluation of bone mineral density (BMD) at baseline and periodically thereafter for women undergoing therapy that lowers sex hormones.9 The latest guidelines from the American Society of Clinical Oncology (ASCO) indicate that postmenopausal women and patients on ovarian suppression who receive adjuvant systemic treatment should be considered for bisphosphonate therapy, such as intravenous (IV) zoledronic acid (4 mg every 6 months) or oral clodronate (1600 mg daily).10 Supplementation to reach 1200 mg calcium (total includes dietary sources) and 800 IU vitamin D daily is recommended for all patients.9
Approximately 45–50% of patients on AIs experience musculoskeletal symptoms, including arthralgia, increased tendon thickness, and carpal tunnel syndrome. These side effects are a common cause of therapy discontinuation.11 Although there is no definitive treatment for these symptoms, exercise, massage therapy, acupuncture, and nonsteroidal antiinflammatory drugs (NSAIDs) have demonstrated varying degrees of benefit.12 Switching from one AI to another often can result in better tolerance and compliance, with 72% of patients continuing on letrozole for six months after discontinuing anastrozole due to musculoskeletal symptoms.13
The decrease in circulating estrogen on AI therapy can lead to sexual dysfunction, including vaginal dryness and atrophy, cystitis, vaginitis, painful intercourse, and decreased libido. These side effects are underreported and cause patients significant emotional and physical distress. It is recommended that sexual dysfunction be actively discussed with patients; potential interventions include education, sexual aids (eg, lubricants, lidocaine preparations), sexual counseling, non-hormonal medications, and dietary supplements. Low-dose vaginal estrogen may be considered for refractory cases.14
Cyclin-Dependent Kinases 4 and 6 Inhibitors
Endocrine therapy is the mainstay treatment for patients with metastatic hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2–) breast cancer. The advent of cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors such as abemaciclib, palbociclib, and ribociclib, in combination with endocrine therapy to overcome endocrine resistance, has been a breakthrough for first-line treatment of postmenopausal women with HR+ metastatic disease. The use of a CDK4/6 inhibitor with letrozole or anastrozole has been shown to increase progression-free survival to 24–25 months, compared with 14–15 months for endocrine therapy alone.
Up to 92% of patients receiving CDK4/6 inhibitors experience neutropenia and lymphopenia, with anemia and thrombocytopenia occurring less commonly. Unlike traditional chemotherapy, which causes apoptosis of bone marrow cells, CDK4/6 inhibitors merely arrest dividing cells in the bone marrow, and neutropenia is rapidly reversed within 48 hours of therapy cessation without the need for stimulating factors.22–24
Please see the following information on the management of adverse events with specific CDK4/6 inhibitors.
Palbociclib25
| Dose level | Dose |
| Recommended starting dose | 125 mg/day |
| First dose reduction | 100 mg/day |
| Second dose reduction | 75 mg/day |
| Further dose reductions below 75 mg/day | Discontinue |
Dose modification for hematologic toxicities
- Grades 1 and 2: no adjustment required
- Grade 3:
- Day 1 of cycle: withhold palbociclib and repeat complete blood count monitoring within 1 week. When recovered to grade ≤2, start the next cycle at the same dose.
- Day 15 of first 2 cycles: If grade 3 on day 15, continue at current dose to complete cycle and repeat complete blood count on day 22. If grade 4 on day 22, see grade 4 dose modification guidelines below.
- Consider dose reduction in cases of prolonged (>1 week) recovery from grade 3 neutropenia or recurrent grade 2 neutropenia on day 1 of subsequent cycles.
- If absolute neutrophil count [ANC] 500–<1000 mm3 + fever or infection: hold palbociclib until recovery to grade ≤2 and reduce to next lowest dose
- Grade 4: hold palbociclib until recovery to grade ≤2 and reduce to next lowest dose
Dose modification for non-hematologic toxicities
- Grades 1 and 2: no adjustment required
- Grade 3 or greater: hold until symptoms resolve to grade 2, if not considered a safety risk; if there is a safety risk, hold until grade 1 and resume at next lower dose.
Ribociclib26
| Dose level | Dose |
| Starting dose | 600 mg/day |
| First dose reduction | 400 mg/day |
| Second dose reduction | 200 mg/day |
| Further dose reduction below 200 mg/day | Discontinue |
Dose modification with neutropenia
- Grade 1 or 2: no adjustment required
- Grade 3 (afebrile): hold until recovery to grade ≤2 and resume at same dose; if toxicity recurs at grade 3, dose interruption until recovery, then resume with decreased dose with next cycle
- Grade 3 (febrile) or grade 4: hold until recovery to grade ≤2; decrease dose with next cycle
Dose modification with QT prolongation
- QTcF >480 msec: hold dose until recovery to <481 and reduce dose; if recurs, interrupt dose and resume at next lower dose once resolved
- QTcF >500 msec: hold dose if >500; resume at next lower dose when QTcF <481
- QTcF >500 msec or changed by 60 msec from baseline: permanently discontinue if also associated with Torsades de Pointes, polymorphic ventricular tachycardia, unexplained syncope, or signs/symptoms of serious arrhythmia
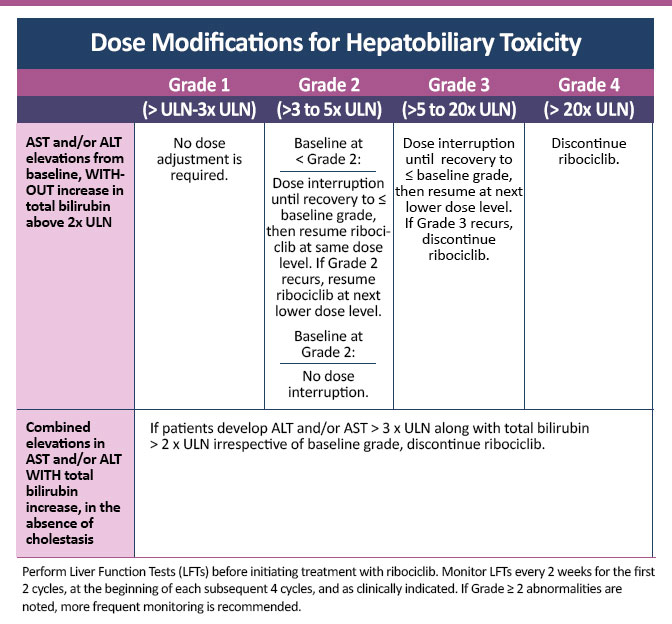
Dose Modifications for Hepatobiliary Toxicity
Abemaciclib27
|
Dose level |
Dose in combination with fulvestrant or an aromatase inhibitor |
Dose for monotherapy |
| Recommended starting dose | 150 mg twice daily | 200 mg twice daily |
| First dose reduction | 100 mg twice daily | 150 mg twice daily |
| Second dose reduction | 50 mg twice daily | 100 mg twice daily |
| Third dose reduction | Not applicable | 50 mg twice daily |
Dose modification for hematologic toxicities
- Grade 1 or 2: no dose modification required
- Grade 3: hold dose until toxicity resolves to ≤grade 2; dose reduction not required
- Grade 3 recurrent or grade 4: hold dose until toxicity resolves to ≤grade 2; resume at next lower dose
- If blood-cell growth factors are required, hold abemaciclib dose for at least 48 hours after last dose of blood-cell growth factor and until toxicity resolves to ≤grade 2; resume at next lower dose (if not already done)
Dose modification for diarrhea
- Grade 1: no dose modification required
- Grade 2: if toxicity does not resolve within 24 hours to ≤grade 1, suspend abemaciclib dose until resolution; no dose modification required
- Persistent or recurrent grade 2 despite maximal supportive measures: suspend dose until toxicity resolves to ≤grade 1; resume at next lower dose
- Grades 3 or 4 or requiring hospitalization: suspend dose until toxicity resolves to ≤grade 1; resume at next lower dose
Dose modification for hepatotoxicity
- Grade 1 (alanine aminotransferase [ALT]/aspartate aminotransferase [AST] > upper limit of normal [ULN] to 3x ULN, total bilirubin ≤2 ULN): no dose modification required
- Grade 2 (ALT/AST >3 to 5x ULN, total bilirubin ≤2 ULN): no dose modification required
- Persistent or recurrent grade 2 or grade 3 (ALT/AST >5 to 20x ULN) without increase in total bilirubin >2x ULN: suspend dose until toxicity resolves to baseline or grade 1; resume at next lower dose
- Grade 4 (ALT/AST >20x ULN): discontinue
- Elevation in AST and/or ALT >3x ULN with total bilirubin >2x ULN (without cholestasis): discontinue
Dose modification for other toxicities
- Grades 1 and 2: no adjustment required
- Persistent or recurrent grade 2 that does not resolve with maximal supportive measures within 7 days to baseline or grade 1: hold treatment until ≤grade 1 and resume at lower dose
- Grade 3 or 4: hold treatment until ≤grade 1 and resume at lower dose
References
- Aiello Bowles EJ, Boudreau DM, Chubak J, et al. Patient-reported discontinuation of endocrine therapy and related adverse effects among women with early-stage breast cancer. J Oncol Pract.2012;8:e149-e157. Available at https://ascopubs.org/doi/pdf/10.1200/JOP.2012.000543
- Adapted from Pirouzi P, Patrick A, Mekhail M. Slides (2011) are available at www.slideshare.net/alpatric/tamoxifen-presentation-10718324
- Goetz MP, Rae JM, Suman VJ, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol.2005;23:9312-9318. Available at https://ascopubs.org/doi/pdf/10.1200/JCO.2005.03.3266
- Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. 2013;381:805-816. Available at www.thelancet.com/action/showPdf?pii=S0140-6736%2812%2961963-1
- Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 126: management of gynecologic issues in women with breast cancer. Obstet Gynecol.2012;119:666-682. PubMed page at ncbi.nlm.nih.gov/pubmed/?term=ACOG+AND+2012%5Bdp%5D+AND+666-
- Day R; National Surgical Adjuvant Breast and Bowel Project P-1 study. Quality of life and tamoxifen in a breast cancer prevention trial: a summary of findings from the NSABP P-1 study. Ann NY Acad Sci.2001;949:143-150. Available at https://nyaspubs.onlinelibrary.wiley.com/doi/pdf/10.1111/j.1749-6632.2001.tb04012.x
- Paganini-Hill A, Clark LJ. Eye problems in breast cancer patients treated with tamoxifen. Breast Cancer Res Treat.2000;60:167-172. Abstract at ncbi.nlm.nih.gov/pubmed/?term=paganini-Hill%5B1au%5D+AND+2000%5Bdp%5D+AND+167
- Amir E, Seruga B, Niraula S, et al. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systemic review and meta-analysis. J Natl Cancer Inst.2011;103:1299-1309. Available at https://academic.oup.com/jnci/article/103/17/1299/2516517
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast Cancer. Version 3.2019. nccn.org/professionals/physician_gls/pdf/breast.pdf
- Dhesy-Thind S, Fletcher GG, Blanchette PS, et al. Use of adjuvant bisphosphonates and other bone-modifying agents in breast cancer: a Cancer Care Ontario and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol.2017;35:2062-2081. Available at https://ascopubs.org/doi/pdf/10.1200/JCO.2016.70.7257
- Beckwée D, Leysen L, Meuwis K, Adriaenssens N. Prevalence of aromatase inhibitor-induced arthralgia in breast cancer: a systemic review and meta-analysis. Support Care Cancer.2017;25:1673-1686. Abstract at ncbi.nlm.nih.gov/pubmed/?term=beckwee%5B1au%5D+AND+2017%5Bdp%5D+AND+1673
- Younus J, Kligman L. Management of aromatase inhibitor-induced arthralgia. Curr Oncol.2010;17:87-90. Available at https://current-oncology.com/index.php/oncology/article/view/473/416;
- Briot K, Tubiana-Hulin M, Bastit L, et al. Effect of a switch of aromatase inhibitors on musculoskeletal symptoms in postmenopausal women with hormone-receptor-positive breast cancer: the ATOLL (articular tolerance of letrozole) study. Breast Cancer Res Treat.2010;120:127-134. Abstract at ncbi.nlm.nih.gov/pubmed/?term=briot%5B1au%5D+AND+2010%5Bdp%5D+AND+127
- Carter J, Lacchetti C, Andersen BL, et al. Interventions to address sexual problems in people with cancer: American Society of Clinical Oncology clinical practice guideline adaptation of Cancer Care Ontario guideline. J Clin Oncol.2018;36:492-511. Available at https://ascopubs.org/doi/pdf/10.1200/JCO.2017.75.8995
- Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003;348:2431-2442. Available at nejm.org/doi/full/10.1056/NEJMra023246?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed
- Perez EA. Safety profiles of tamoxifen and the aromatase inhibitors in adjuvant therapy of hormone-responsive early breast cancer. Ann Oncol. 2007;18(supp 8):viii26-viii35. Available at https://academic.oup.com/annonc/article/18/suppl_8/viii26/197125
- Early Breast Cancer Trialists’ Collaborative Group (EBCTG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. 2015;386:1341-1352. Available at www.thelancet.com/action/showPdf?pii=S0140-6736%2815%2961074-1
- Letrozole (Femara®) prescribing information (PI), 2018. Available at https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/Femara.pdf
- Anastrozole (Arimidex®) PI, 2019. Available at https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=acbfaaa9-503c-4691-9828-76a7146ed6de
- Exemestane (Aromasin®) PI, 2018. Available at http://labeling.pfizer.com/ShowLabeling.aspx?id=523
- Tamoxifen (Soltamox®) PI, 2018. Available at http://soltamox.com/wp-content/uploads/2016/07/Soltamox-FDA-Approved-Package-Insert.pdf
- Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol.2017;35:3638-3646. Available at https://ascopubs.org/doi/pdf/10.1200/JCO.2017.75.6155
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med.2016;375:1738-1748. Available at nejm.org/doi/10.1056/NEJMoa1609709?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dwww.ncbi.nlm.nih.gov
- Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med.2015;373:209-219. Available at nejm.org/doi/pdf/10.1056/NEJMoa1505270?articleTools=true
- Palbociclib (Ibrance®) PI, 2019. Available at http://labeling.pfizer.com/ShowLabeling.aspx?id=2191#section-2.2
- Ribociclib (Kisqali®) PI, 2019. Available at pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/kisqali.pdf
- Abemaciclib (Verzenio®) PI, 2019. Available at http://uspl.lilly.com/verzenio/verzenio.html#pi
