Overview
Epidemiology
- In 2019, it is estimated that 268,600 individuals in the United States (US) will receive a new breast cancer diagnosis. Approximately 99% of these patients will be female.1
- It is estimated that 15.2% of new cancer cases diagnosed in women in 2019 will be breast cancer.2
- The 5-year overall survival for patients with metastatic breast cancer is approximately 27%, with a median overall survival of 2–3 years.1,3
- There will be approximately 42,260 deaths from breast cancer in the US in 2019.1
- Due to early detection and individualized treatment, the death rate for breast cancer has decreased over the past 20 years.1
- A woman’s approximate lifetime risk of developing breast cancer is 12.8%.2
- Data support the correlation between a strong nurse-patient relationship and a positive influence on patient satisfaction, improvements in adherence, health literacy, and better quality of life.4,5
Pathophysiology
The hormone estrogen promotes the growth and survival of normal and cancerous breast epithelial cells by binding to the estrogen receptor (ER). Activation of the ER promotes the expression of genes responsible for cell division, inhibition of cell death, and formation of new blood vessels. It is estimated that approximately 70% of breast cancers overexpress the ER, and interrupting the activity of this receptor blocks the cell signaling necessary for breast cancer growth and division. There are three classes of drugs that interrupt the estrogen-dependent pathways of breast cancer. Selective ER modulators (SERMs), such as tamoxifen and raloxifene, block the binding of estrogen to the ER. Selective ER downregulators, such as fulvestrant, decrease the number of ERs a cell produces, significantly reducing the cell’s ability to respond to estrogen. Aromatase inhibitors, such as anastrozole, letrozole, and exemestane, prevent the production of estrogen.6
Actively dividing cells pass through a series of stages—the cell cycle—as they grow and duplicate their deoxyribonucleic acid (DNA) in preparation for division. The cell cycle is controlled by a cascade of proteins and kinases that check to see if the cell is ready for division before proceeding to the next phase of the cell cycle. Tumors develop from an unregulated division of cells, and many mutations in cell-cycle proteins have been associated with an increased risk of cancer development.7,8
Tumor-suppressor genes, such as the retinoblastoma (Rb) protein, regulate the cell cycle and normally inhibit cell division. If the enzymes cyclin-dependent kinase 4/6 (CDK4/6) inactivate the Rb protein, the cell begins to duplicate its DNA, and the cell cycle is initiated. In a non-cancerous cell, CDK4/6 is only activated when the cell receives signals indicating division is necessary. In cancer, tumor-suppressor genes such as Rb may be inactivated by a mutation, or kinases such as CDK4/6 may be overexpressed. CDK4/6 inhibitors such as abemaciclib, palbociclib, and ribociclib work by blocking the activity of CDK4/6, preventing the inactivation of Rb and arresting the cell cycle.7–9
The combination of a CDK4/6 inhibitor with endocrine therapy in patients with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2–) advanced/metastatic breast cancer has demonstrated superiority over endocrine therapy alone across multiple patient subtypes, regardless of age, performance status, menopausal status, extent of disease, or race. Knowledge of the clinical trial data on dosing, side-effect management, study endpoints, monitoring parameters, and patient population characteristics is important since these considerations will be factors in choosing which of these potent agents to use in this difficult to treat malignancy.9
Person-Centered Care
Systemic treatment of early and advanced breast cancer is mainly driven by the biological characteristics of the disease, particularly by expression of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). Hormone receptor-positive (HR+) tumors can be treated with endocrine therapy and have a better prognosis than other breast cancer subtypes. HER2-positive tumors tend to grow and spread faster than other breast cancers and are more likely to benefit from targeted therapy against the HER2 protein. Triple-negative breast cancers do not overexpress hormone receptors or the HER2 protein, ie, they are ER–/PR–/HER2–. Chemotherapy is the standard treatment for this subtype of breast cancer.
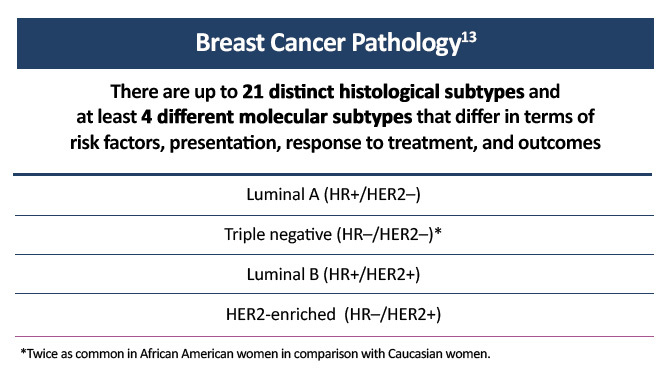
Figure 1. Molecular subtypes of breast cancer.
Endocrine Resistance
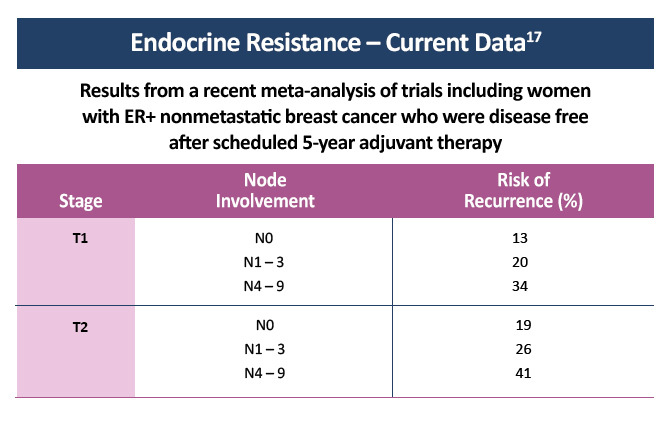
Hormone receptor-positive (HR+) breast cancer responds to anti-estrogen therapy and has a better prognosis than other types of breast cancer. Approximately 50% of patients with early stage estrogen receptor-positive (ER+) cancer obtain benefit from adjuvant endocrine monotherapy. However, resistance to endocrine therapy leading to the recurrence of early stage ER+ breast cancer is not uncommon and is inevitable in the development of advanced disease.15 Nearly all breast cancer deaths are caused by metastases rather than by the primary tumor. Although HR+ tumors are highly sensitive to endocrine therapy, approximately 20–40% of breast cancer patients eventually develop recurrences in distant organs, often years after diagnosis. This phenomenon of later metastasis is especially pronounced in ER+ breast cancers, suggesting that these tumors remain dormant for extended periods of time or proliferate slowly despite adjuvant therapies.6
Many of the ER+ tumors that show resistance to endocrine monotherapy have functional retinoblastoma proteins and cyclin-dependent kinase 4 and 6 (CDK4/6) pathways, making these patients good candidates for CDK4/6 inhibitor treatment.16 Phase 3 clinical trials have shown that the combination of CDK4/6 inhibitors and endocrine therapy for advanced ER+ breast cancer improves progression-free survival when compared with endocrine therapy alone. The combination of CDK4/6 inhibitors and endocrine therapy is now the standard of care as first-line therapy for advanced ER+ breast carcinoma in many countries.15
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. Available at https://onlinelibrary.wiley.com/doi/full/10.3322/caac.21551
- National Cancer Institute. Cancer Stat Facts: female breast cancer. Available at https://seer.cancer.gov/statfacts/html/breast.html
- American Cancer Society. Breast Cancer Facts and Figures 2019-2020. Available at cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2019-2020.pdf
- Albert US, Zemlin C, Hadji P, et al. The impact of breast care nurses on patients’ satisfaction, understanding of the disease, and adherence to adjuvant endocrine therapy. Breast Care (Basel).2011;6:221-226. Available at karger.com/Article/Pdf/329006
- Cardoso F, Costa A, Senkus E, et al. 3rd ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 3). Ann Oncol.2017;28:16-33. Available at https://academic.oup.com/annonc/article/28/1/16/2660178
- Fabian CJ. The what, why and how of aromatase inhibitors: hormonal agents for treatment and prevention of breast cancer. Int J Clin Pract.2007;61:2051-2063. Available at https://onlinelibrary.wiley.com/doi/epdf/10.1111/j.1742-1241.2007.01587.x
- Barnum KJ, O’Connell MJ. Cell cycle regulation by checkpoints. Methods Mol Biol.2014;1170:29-40. Available at ncbi.nlm.nih.gov/pmc/articles/PMC4990352/pdf/nihms734418.pdf
- Deshpande A, Sicinski P, Hinds PW. Cyclins and cdks in development and cancer: a perspective. 2005;24:2909-2915. Available at nature.com/articles/1208618.pdf
- Knudsen ES, Witkiewicz AK. The strange case of CDK4/6 inhibitors: mechanisms, resistance, and combination strategies. Trends Cancer.2017;3:39-55. Available at ncbi.nlm.nih.gov/pmc/articles/PMC5347397/pdf/nihms834450.pdf
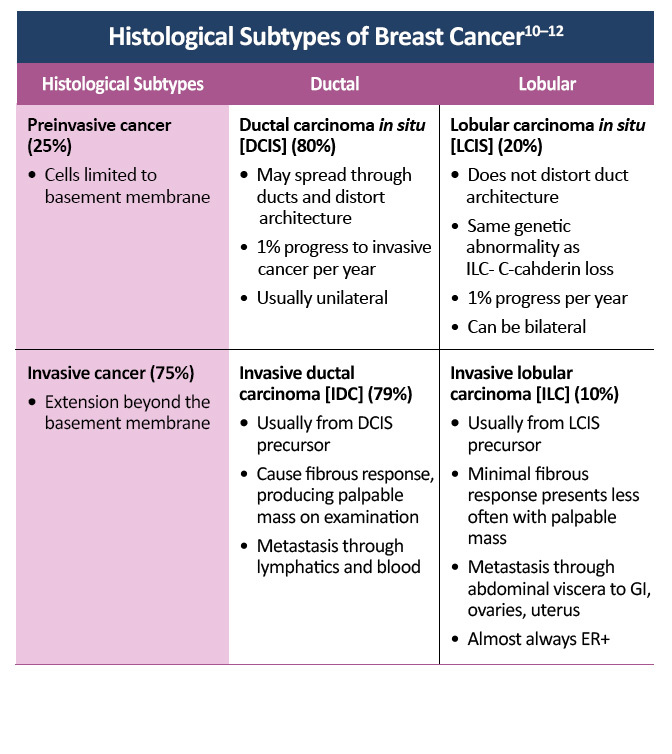
- Henry-Tillman RS, Klimberg VS. In situ breast cancer. Curr Treat Options Oncol.2000;1:199-209. Abstract at ncbi.nlm.nih.gov/pubmed/?term=henry-tillman%5B1au%5D+AND+2000%5Bdp%5D+AND+199-
- Hergueta-Redondo M, Palacios J, Cano A, Moreno-Bueno G. “New” molecular taxonomy in breast cancer. Clin Transl Oncol. 2008;10:777-785. Abstract at ncbi.nlm.nih.gov/pubmed/?term=hergueta-redondo%5B1au%5D+AND+2008%5Bdp%5D+AND+777-
- Sims AH, Howell A, Howell SJ, Clarke RB. Origins of breast cancer subtypes and therapeutic implications. Nat Clin Pract Oncol. 2007;4:516-525. Abstract at ncbi.nlm.nih.gov/pubmed/?term=sims%5B1au%5D+AND+2007%5Bdp%5D+AND+516-
- American Cancer Society. Breast Cancer Facts & Figures 2017. Available at cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf
- Adapted from Malhotra GK, Zhao X, Band H, Band V. Histological, molecular and functional subtypes of breast cancers. Cancer Biol Ther. 2010;10:955-960. Available at tandfonline.com/doi/pdf/10.4161/cbt.10.10.13879?needAccess=true
- Portman N, Alexandrou S, Carson E, et al. Overcoming CDK4/6 inhibitor resistance in ER positive breast cancer. Endocr Relat Cancer.2019;26:R15-R30. Available at https://erc.bioscientifica.com/view/journals/erc/26/1/ERC-18-0317.xml
- Ertel A, Dean JL, Rui H, et al. RB-pathway disruption in breast cancer: differential association with disease subtypes, disease-specific prognosis and therapeutic response. Cell Cycle.2010;9:4153-4163. Available at tandfonline.com/doi/pdf/10.4161/cc.9.20.13454?needAccess=true
- Pan H, Gray R, Braybrooke J, et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med.2017;377:1836-1846. Available at nejm.org/doi/10.1056/NEJMoa1701830?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dwww.ncbi.nlm.nih.gov
